Treatment
Volumising with hyaluronic acid fillers
Recovery Time
4 weeks
Longevity
9-12 months
Our Temple Lift procedure is designed to enhance and elevate your natural beauty with precision and professionalism. At Beautiphi, we pride ourselves on our expertise and skill in transcending the ordinary.
Our seasoned practitioners are devoted to sculpting face contours with delicate care, giving results that symbolise timeless beauty, guided by devotion to the highest standards. Beautiphi-Temple Lift will take you on a rejuvenating experience like no other.
How does temple lift work?
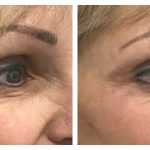
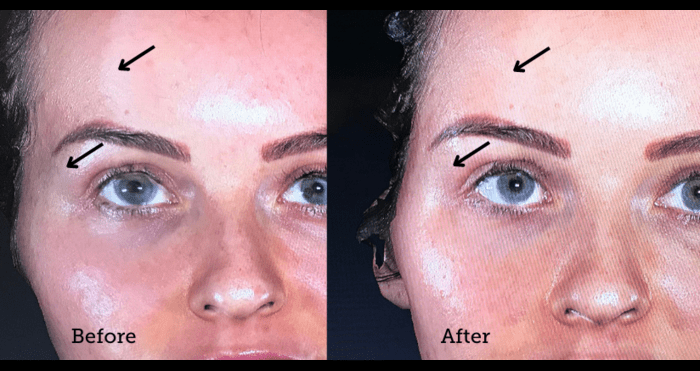
Belotero Intense® dermal filler is made of a material called hyaluronic acid, or HA. HA naturally exists as a component of your skin. By binding to water, it plumps and fills in hollow temples, allowing immediate and smooth correction.
Temple augmentation with dermal filler
Temple lift also known as temple augmentation can help stop the loss of volume in the temple and achieve a more balanced and youthful appearance. Although temples are a critical area for volume restoration in ageing faces, they are not as obvious as other facial folds or wrinkles and maybe sometimes be overlooked by the patient. However, with dermal fillers, augmentation of the temples can alter the upper and mid-face and stop loss of volume which results in a skinny, wasted appearance.
When discussing treatment options for managing volume loss in the face, Stacey recommends considering the potential benefit of temporal fossa augmentation with dermal fillers.
Stacey recommends Belotero Intense ® dermal filler.
FAQs On Temple Lift
Instantly! and individual results may vary.
As with all needle injections, there can be some discomfort associated with the procedure. Stacey can discuss ways to manage any discomfort during the procedure.
Belotero Intense® dermal filler is injected into the skin in a simple, quick procedure using a fine micro-cannula. It can even be done over your lunch hour.
Belotero Intense® dermal filler is approved in New Zealand and Australia, and has undergone testing in clinical studies to prove its safety for use in the human body.
Just like any injection, you may experience mild irritation, swelling, itching, redness, bruising or tenderness at the injection site.
Belotero® is hyaluronic acid dermal filler. It is used to improve the look of facial fold and wrinkles and restore volume, including contouring, shaping and volumising of the lips. Always read the label and consult your Healthcare Professional for more information. This medical device must be administered by a Healthcare Professional. You should tell your practitioner and avoid treatment with BELOTERO® if you: have had an allergic reaction to any of the ingredients; if you tend to develop keloids or heavy scars; have any bleeding disorders, poor wound healing, inflamed or infected skin, or general infection; are under the age of 18; are pregnant or breastfeeding. Please inform your practitioner of any diseases you have or have had. These include in particular cardiovascular diseases, autoimmune diseases, diabetes, epilepsy, liver or kidney problems, skin infections or severe allergies. If you take medication or vitamins, have had previous cosmetic procedures, or have been treated with other implants, please inform your practitioner. Copyright © 2019. Pharmacy Retailing NZ Limited t/a Health Care Logistics (HCL). 58 Richard Pearse Drive, Mangere, Auckland 2022 All rights reserved. Belotero ® and Merz Aesthetics are registered trademarks of Merz Pharma GMbH & Co. KGaA
TEOSYAL RHA® 1, TEOSYAL RHA® 2, TEOSYAL RHA® 3, TEOSYAL RHA® 4, TEOSYAL® PURESENSE REDENSITY 1, TEOSYAL® PURESENSE REDENSITY 2, TEOSYAL PURESENSE KISS ®, and TEOSYAL® PURESENSE ULTRA DEEP are trademark of the firm TEOXANE SA. These products are gel that contains hyaluronic acid, and 0.3% by weight of lidocaine hydrochloride (local anesthetic can induce a positive reaction to anti-doping tests). In the case of known hypersensitivity to lidocaine and/or amide local anaesthetic agents, we recommend not use lidocaine-containing products and please refer to products without lidocaine. They are class III medical device and are regulated health product bearing the CE marking (CE2797) under this regulation.
Local manifestations: inflammatory reactions (erythema, oedema, pain at the point of injection), haematomas, itching, temporary loss of sensitivity around the injected area, dyschromia, abscesses, indurations, nodules (possibly granulomas).
General manifestations: immediate hypersensitivity up to anaphylactic shock, migration of the implant.
Rare but serious adverse events associated with the intravascular injection of soft tissue fillers in the face have been reported and include temporary or permanent vision impairment or blindness, skin necrosis and stroke.
Please consult your physician or pharmacist for more information.
Please refer to instructions for use